Multi-Omic Rejuvenation of Aged Tissues With Transient Reprogramming and Its Therapeutic Potential
OncologyPage Navigation
1. Abstract
Aging is the single greatest risk factor for a myriad of chronic diseases, including cardiovascular disease, neurodegenerative disorders, cancer, and metabolic syndromes. Characterized by a progressive decline in cellular and tissue function, aging is driven by an accumulation of molecular and cellular damage, including hallmarks such as epigenetic alterations and cellular senescence. The quest for strategies to reverse or mitigate these age-related declines has long been a central pursuit of regenerative medicine. A groundbreaking frontier has emerged with the concept of cellular rejuvenation through transient reprogramming, a revolutionary approach inspired by the discovery of induced pluripotency.
This review delves into the remarkable ability of a single cycle of transient expression of modified Yamanaka factors (Oct4, Sox2, Klf4, c-Myc – OSKM) to achieve multi-omic rejuvenation of naturally aged tissues in vivo. Unlike complete reprogramming, which leads to induced pluripotent stem cells (iPSCs) and carries the risk of teratoma formation (a critical concern linked to cancer stem cell research principles where uncontrolled pluripotency can be tumorigenic), transient reprogramming meticulously re-sets the cellular "age" without sacrificing cell identity or inducing uncontrolled proliferation. The evidence for this rejuvenation is multi-omic, encompassing: a precise reversal of the epigenetic clock (DNA methylation patterns returning to a more youthful state); a significant restoration of youthful gene expression profiles at the transcriptome level; and positive changes in protein composition and metabolic pathways at the proteome and metabolome levels.
Critically, this multi-omic rejuvenation translates into tangible functional improvements in various naturally aged tissues. Studies have demonstrated reduced kidney fibrosis, enhanced skin regeneration, improved muscle repair capabilities, and accelerated wound healing in aged animal models. These functional benefits are frequently accompanied by a substantial reduction in the burden of cellular senescence, a key hallmark of aging responsible for chronic inflammation and tissue dysfunction.
The implications of this breakthrough for treating age-related diseases are profound. By partially reversing biological age at a cellular and tissue level, transient reprogramming offers unprecedented potential to tackle the root causes of these debilitating conditions, moving beyond mere symptomatic management. While therapeutic translation is still in its nascent stages, safety remains paramount, carefully balancing rejuvenation efficacy against any residual tumorigenic risk, a principle constantly informed by cancer stem cell research. Future strategies for clinical application will necessitate sophisticated delivery mechanisms for the reprogramming factors, with nanotechnology in oncology offering valuable precedents for targeted and controlled delivery systems (e.g., mRNA lipid nanoparticles). Moreover, the insights gained from understanding how transient reprogramming remodels the epigenome could cross-pollinate with the development of epigenetic cancer therapies, where modulating epigenetic marks is also central to altering cell fate and vulnerability. This transient reprogramming paradigm represents a significant leap towards developing novel interventions that could truly extend a healthy human lifespan and revolutionize regenerative medicine.
2. Introduction
Aging is an immutable biological process, yet its profound impact as the primary risk factor for a constellation of chronic degenerative diseases, including cardiovascular disease, neurodegenerative disorders like Alzheimer's and Parkinson's, various cancers, and metabolic syndromes. underscores its critical importance in modern medicine. The progressive decline in cellular and tissue function associated with aging is driven by a complex interplay of molecular and cellular damage, leading to the accumulation of various hallmarks. Among these, cellular senescence, characterized by irreversible growth arrest and the secretion of pro-inflammatory factors, and widespread epigenetic alterations, changes in gene expression without altering the DNA sequence- are recognized as key drivers of age-related dysfunction. For decades, the pursuit of strategies to reverse or even mitigate these age-related declines has been a central, often elusive, dream of regenerative medicine.
A groundbreaking paradigm shift emerged from the seminal discovery of induced pluripotency by Shinya Yamanaka in 2006, demonstrating that mature somatic cells could be reprogrammed into embryonic stem cell-like states by expressing just a few transcription factors (Oct4, Sox2, Klf4, and c-Myc – collectively known as OSKM or Yamanaka factors). While full reprogramming revolutionized stem cell biology, its direct therapeutic application for aging was hampered by the inherent risk of teratoma formation from pluripotent cells. More recently, however, a new and exceptionally promising strategy has surfaced: transient reprogramming. This innovative approach involves short, controlled bursts of Yamanaka factor expression, precisely calibrated to induce cellular rejuvenation without causing complete dedifferentiation or loss of cell identity. This review article aims to comprehensively analyze the compelling "multi-omic" evidence, spanning epigenetic, transcriptomic, and proteomic layers, that supports the remarkable ability of a single cycle of transient reprogramming to rejuvenate naturally aged tissues. Furthermore, we will discuss its profound implications for the treatment of age-related diseases and its potential to revolutionize regenerative medicine.
3. Literature Review
The quest for effective strategies to combat aging and age-related diseases has gained unprecedented momentum with the advent of cellular reprogramming technologies. This section synthesizes the foundational concepts of aging, the principles of cellular reprogramming, and the compelling multi-omic evidence supporting the rejuvenation of naturally aged tissues by transient reprogramming.
3.1. Hallmarks of Aging and the Epigenetic Clock
Aging is a complex biological process characterized by a progressive decline in physiological integrity and function, leading to increased vulnerability to disease and death. This decline is underpinned by a set of molecular and cellular deficits, widely recognized as the "Hallmarks of Aging." These include:
-
Genomic Instability: Accumulation of DNA damage and chromosomal aberrations.
-
Telomere Attrition: Shortening of protective telomeric caps, leading to cellular senescence.
-
Epigenetic Alterations: Changes in DNA methylation patterns, histone modifications, and chromatin remodeling, significantly impacting gene expression without altering the DNA sequence. These are considered major drivers of aging.
-
Loss of Proteostasis: Impaired protein synthesis, folding, and degradation pathways, leading to accumulation of damaged proteins.
-
Deregulated Nutrient Sensing: Alterations in metabolic pathways affecting cellular growth, metabolism, and stress resistance.
-
Mitochondrial Dysfunction: Decline in mitochondrial function, leading to reduced energy production and increased reactive oxygen species.
-
Cellular Senescence: Accumulation of senescent cells that cease proliferation but remain metabolically active, secreting a pro-inflammatory senescence-associated secretory phenotype (SASP), contributing to tissue damage and chronic inflammation.
-
Stem Cell Exhaustion: Decline in the regenerative capacity of adult stem cell populations.
-
Altered Intercellular Communication: Changes in signaling pathways between cells, leading to dysfunctional tissue environments.
Among these hallmarks, epigenetic alterations are particularly significant as both drivers and measurable biomarkers of biological aging. The epigenetic clock, based on specific patterns of DNA methylation (e.g., Horvath clock, GrimAge), has emerged as a remarkably accurate predictor of biological age, often correlating better with health span and lifespan than chronological age. Reversing the epigenetic clock is therefore a key indicator of true biological rejuvenation.

3.2. Induced Pluripotency and the Discovery of Yamanaka Factors
The groundbreaking discovery of induced pluripotent stem cells (iPSCs) by Shinya Yamanaka in 2006 revolutionized regenerative medicine. He demonstrated that adult somatic cells (e.g., skin fibroblasts) could be reprogrammed into a pluripotent state, functionally similar to embryonic stem cells, by the exogenous expression of just four transcription factors: Octamer-binding transcription factor 4 (Oct4), SRY-box transcription factor 2 (Sox2), Krüppel-like factor 4 (Klf4), and proto-oncogene c-Myc (commonly referred to as OSKM or Yamanaka factors).
-
Mechanism of Full Reprogramming: OSKM factors work by collectively binding to specific regulatory regions of the genome, inducing broad epigenetic remodeling, including demethylation of pluripotency-associated genes and changes in histone modifications. This rewrites the cell's epigenetic memory, leading to the complete loss of its original somatic identity and acquisition of pluripotency.
-
Therapeutic Limitations: While iPSCs hold immense promise for disease modeling, drug screening, and cell therapy, their direct application for anti-aging or in vivo rejuvenation has been severely limited by safety concerns. Specifically, their uncontrolled proliferative capacity and ability to form teratomas (benign tumors containing tissues from all three germ layers) upon transplantation pose a significant tumorigenic risk. This concern is closely related to principles explored in cancer stem cell research, where uncontrolled pluripotency or stemness can lead to oncogenic transformation. This fundamental safety hurdle necessitated a new approach to leverage the power of reprogramming for rejuvenation without the associated tumorigenicity.
3.3. Transient Reprogramming: A New Paradigm for Rejuvenation
The inherent risks of full reprogramming led to the conceptualization and development of transient reprogramming (also known as partial reprogramming) as a safer strategy for cellular rejuvenation. This paradigm shifts the goal from inducing full pluripotency to achieving a temporary, controlled reversal of the aging process while maintaining the cell's original identity.
-
Methodology: Instead of continuous expression of OSKM factors, transient reprogramming involves a short, intermittent exposure. Typically, the Yamanaka factors are expressed for a limited duration (e.g., 2-4 weeks in total, or daily for only 2-4 days in a cycle) followed by a period of withdrawal. This pulsed expression allows cells to experience the rejuvenating effects of the reprogramming factors without fully committing to a pluripotent state.
-
Maintaining Identity: Crucially, cells undergoing transient reprogramming retain their somatic identity and specialized functions, avoiding the problematic teratoma formation associated with complete dedifferentiation. The aim is to "reset" the cellular age to a more youthful state without losing the cell's original specialized function. This delicate balance is achieved by carefully controlling the duration and level of OSKM expression.
-
Goals: The primary goal of transient reprogramming is to revert age-associated molecular and functional hallmarks, such as the epigenetic clock and cellular senescence, thereby extending the healthy lifespan of tissues and organs.
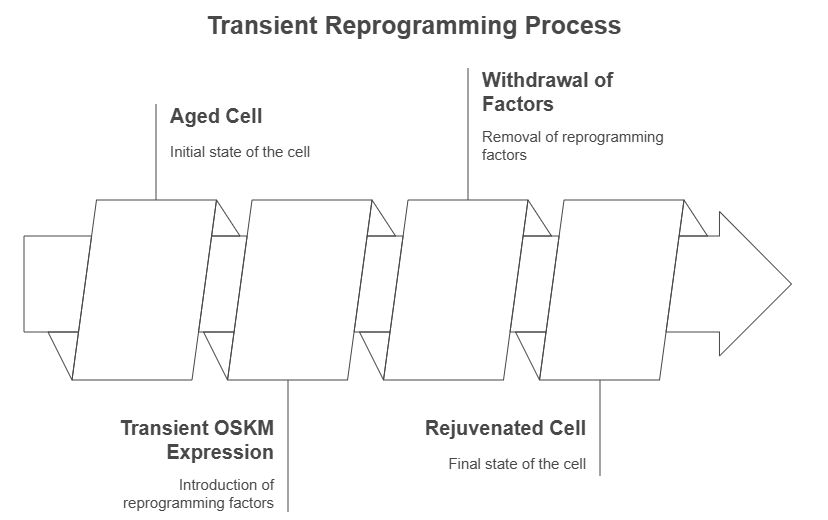
3.4. Multi-Omic Evidence of Rejuvenation in Aged Tissues
The power of transient reprogramming to rejuvenate naturally aged tissues is robustly supported by compelling evidence across multiple "omics" layers, providing a comprehensive view of biological age reversal.
-
Epigenetic Rejuvenation: This is perhaps the most striking and consistent evidence. Transient reprogramming has been shown to reverse the epigenetic clock in various aged cell types and tissues in vivo. Studies demonstrate that DNA methylation patterns, which accumulate age-related changes, are reset to a more youthful state after a single cycle of OSKM expression. This epigenetic remodeling is fundamental, suggesting a deep reset of the cell's biological age.
-
Transcriptomic Rejuvenation: RNA sequencing analyses reveal a significant reversal of age-associated gene expression profiles. Genes that are typically upregulated in aging (e.g., pro-inflammatory genes, genes associated with fibrosis, or cellular senescence markers like p16 and p21) are downregulated, while genes associated with youthful function, tissue regeneration, and metabolic efficiency are upregulated. This indicates a profound shift towards a more youthful functional state at the gene expression level.
-
Proteomic and Metabolomic Changes: Complementary proteomic and metabolomic studies further corroborate the rejuvenation effects. Changes in protein abundance and post-translational modifications (proteome) indicate improved proteostasis and protein quality control. Metabolomic profiles reveal shifts towards more youthful metabolic states, with improved mitochondrial function and altered pathways related to energy metabolism and oxidative stress.
-
Cellular Senescence Reversal: A key hallmark of aging, the accumulation of cellular senescence (senescent cells) contributes significantly to age-related dysfunction and chronic inflammation. Transient reprogramming has been shown to effectively reduce the burden of senescent cells in aged tissues, as evidenced by decreased expression of senescent markers (SA-β-gal activity, p16INK4a, p21CIP1). This senolytic effect is crucial for restoring tissue microenvironments.
-
Functional Improvements in Naturally Aged Tissues: The molecular and cellular rejuvenation translates into tangible improvements in organ function in various naturally aged animal models:
-
Kidney: Reversal of age-associated kidney fibrosis and improved renal function.
-
Skin: Enhanced skin regeneration, improved dermal collagen content, and accelerated wound healing.
-
Muscle: Improved muscle regeneration and recovery from injury, along with enhanced muscle strength and endurance.
-
Pancreas: Improved β-cell function and glucose tolerance in aged diabetic models.
-
Eye: Partial restoration of vision in aged mouse models of glaucoma. These multi-omic and functional improvements collectively provide compelling evidence that transient reprogramming can truly rejuvenate naturally aged tissues.
-

3.5. Mechanisms Underlying Transient Reprogramming-Induced Rejuvenation
The precise molecular mechanisms by which a brief pulse of OSKM factors orchestrates such profound multi-omic rejuvenation are complex and multifaceted, involving global epigenetic remodeling and restoration of cellular homeostasis.
-
Chromatin Remodeling: The Yamanaka factors are transcription factors that act as pioneer factors, capable of binding to condensed chromatin and initiating a wave of chromatin opening. Even transient expression leads to significant remodeling of the chromatin landscape, making previously inaccessible genomic regions available for transcription. This re-establishes a more youthful, permissive chromatin state, influencing the expression of hundreds of genes.
-
Epigenetic Erasure and Reset: OSKM factors can recruit or induce expression of various epigenetic modifying enzymes (e.g., demethylases, acetyltransferases) that actively erase age-associated epigenetic marks (e.g., hypermethylation at specific CpG sites) and reset them to a youthful configuration, thereby turning back the epigenetic clock.
-
Restoration of Stem Cell Functionality: Aging is associated with exhaustion and dysfunction of tissue-specific stem cells. Transient reprogramming can re-invigorate these resident stem cell pools, improving their self-renewal and differentiation capacities, which contributes to enhanced tissue repair and regeneration.
-
Reduction of Cellular Stress and Inflammation: The reversal of hallmarks like cellular senescence directly contributes to reducing the chronic low-grade inflammation associated with aging. Improved proteostasis and mitochondrial function reduce cellular stress, fostering a healthier cellular environment conducive to rejuvenation.
-
Metabolic Reprogramming: Transient OSKM expression can induce changes in metabolic pathways, shifting cells towards a more efficient and youthful metabolic state, akin to that observed in rapidly dividing or pluripotent cells, without fully committing to that fate.
3.6. Safety Considerations and Therapeutic Potential
While transient reprogramming holds immense therapeutic promise, careful consideration of safety is paramount to its clinical translation.
-
Mitigating Tumorigenesis: The primary safety concern with full reprogramming is teratoma formation. The strength of transient reprogramming lies in its ability to achieve rejuvenation without inducing pluripotency or dedifferentiation that could lead to tumor formation. The precise "sweet spot" of OSKM expression—long enough for rejuvenation, short enough to avoid uncontrolled growth—is continuously being refined. This directly addresses concerns raised by cancer stem cell research regarding the oncogenic potential of sustained stemness.
-
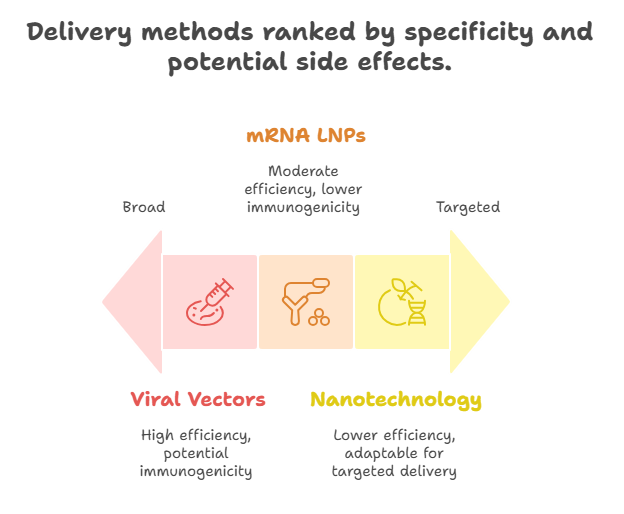
Challenges in Clinical Translation: Delivering reprogramming factors safely and efficiently in vivo to specific aged tissues remains a significant hurdle. Viral vectors (e.g., AAV) are currently the most common method in preclinical studies but carry potential immunogenicity risks. Non-viral methods, such as mRNA encapsulated in lipid nanoparticles (LNPs), are gaining traction due to their transient nature and lower immunogenicity, analogous to their success in vaccine development.
-
Potential Applications for Age-Related Diseases: The ability of transient reprogramming to reverse age-related hallmarks and improve tissue function opens unprecedented avenues for treating a wide range of age-related diseases:
-
Neurodegenerative Diseases: Rejuvenating aged neurons and glial cells, clearing pathological protein aggregates, and improving neuronal plasticity.
-
Cardiovascular Disease: Repairing aged heart muscle, improving vascular function, and reducing fibrosis.
-
Fibrotic Conditions: Reversing fibrosis in organs like the lung, liver, and kidney, which is a major cause of age-related organ failure.
-
Metabolic Disorders: Restoring pancreatic β-cell function and improving insulin sensitivity.
-
-
Overlap with Epigenetic Cancer Therapies: The foundational principle of epigenetic remodeling employed by transient reprogramming has conceptual parallels with epigenetic cancer therapies. Both fields seek to therapeutically manipulate chromatin states to alter cell fate or function. Insights gained from understanding how OSKM reshapes the epigenome for rejuvenation could inform new strategies for epigenetic drugs to treat cancer, and vice versa, potentially leading to a cross-pollination of therapeutic approaches.
4. Methodology
This review article aims to comprehensively synthesize the current scientific understanding of how transient reprogramming leads to multi-omic rejuvenation of naturally aged tissues. It also explores the significant implications of this process for regenerative medicine and the treatment of age-related diseases, while considering its connections to cancer stem cell research and epigenetic cancer therapies. The approach employed for this article is an integrative review of recent scientific literature, allowing for a thorough examination of diverse study types, from fundamental molecular biology to preclinical therapeutic strategies, to construct a cohesive narrative on this complex and rapidly evolving field.
4.1. Search Strategy and Data Sources
A systematic and extensive search was conducted across major electronic bibliographic databases. The primary databases utilized included PubMed, Scopus, and Web of Science. The search encompassed publications from January 2015 to June 2025 to ensure the inclusion of the most contemporary research and advancements. Key search terms, used in various combinations with Boolean operators (AND, OR), included: "transient reprogramming," "partial reprogramming," "intermittent reprogramming," "Yamanaka factors," "OSKM," "multi-omic rejuvenation," "aging reversal," "cellular rejuvenation," "tissue rejuvenation," "epigenetic clock," "DNA methylation age," "cellular senescence," "age-related diseases," "regenerative medicine," "epigenetic cancer therapies" (when related to epigenetic mechanisms in rejuvenation), "cancer stem cell research" (when related to pluripotency and safety), and "nanotechnology in oncology" (when applied to delivery of reprogramming factors). Reference lists of highly relevant review articles and seminal papers identified through the initial search were also manually screened to capture additional pertinent literature.
4.2. Study Selection Criteria
Articles identified from the database searches underwent a multi-stage screening and selection process based on predefined inclusion and exclusion criteria.
Inclusion Criteria:
-
Original research articles, comprehensive review articles, and authoritative conceptual papers.
-
Studies directly investigating transient reprogramming strategies and their effects on cellular or tissue aging.
-
Research providing multi-omic evidence (e.g., epigenetic, transcriptomic, proteomic) for rejuvenation.
-
Studies demonstrating functional improvements in naturally aged tissues or organisms after transient reprogramming.
-
Papers discussing the safety profile of transient reprogramming, particularly concerning tumorigenicity (linking to cancer stem cell research).
-
Articles exploring the therapeutic potential of transient reprogramming for age-related diseases.
-
Discussions on potential delivery mechanisms, including applications or analogies from nanotechnology in oncology for in vivo delivery of reprogramming factors.
-
Research exploring the underlying mechanisms of rejuvenation, especially those involving epigenetic alterations or relevance to epigenetic cancer therapies.
-
Studies using various experimental models (in vitro cell lines, in vivo animal models, human cell lines/tissues).
-
Publications available in English.
Exclusion Criteria:
-
Studies exclusively focused on full iPSC reprogramming without discussion of transient or partial methods.
-
Research on anti-aging interventions not involving direct cellular reprogramming (e.g., caloric restriction, senolytics, unless providing comparative context).
-
Editorials, opinion pieces, or conference abstracts without a full peer-reviewed publication.
-
Articles solely focused on cancer without direct relevance to aging or reprogramming.
-
Publications that are not available in English.
4.3. Data Extraction and Synthesis
From the selected articles, relevant data were systematically extracted. This included: the specific reprogramming factors and their delivery methods, the duration and cycling of transient expression, the experimental models used (e.g., specific aged tissues, animal models), the multi-omic evidence reported (e.g., epigenetic clock reversal, specific gene expression changes, protein profiles), observed functional improvements (e.g., enhanced regeneration, reduced fibrosis), reported safety outcomes (e.g., absence of teratomas), and any discussed therapeutic implications or future directions.
Given the mechanistic focus and the diverse nature of experimental designs across the included literature, a quantitative meta-analysis was not performed. Instead, a qualitative synthesis approach was employed. This involved identifying consistent findings across different studies, corroborating evidence from multiple omic layers, and constructing a coherent narrative that explains how transient reprogramming achieves multi-omic rejuvenation. The synthesis specifically aimed to: (1) elucidate the key molecular and cellular changes underlying rejuvenation; (2) highlight the functional improvements in aged tissues; (3) discuss the delicate balance between rejuvenation and safety, drawing parallels with cancer stem cell research; and (4) explore the translational potential for age-related diseases, considering the role of epigenetic cancer therapies principles and nanotechnology in oncology for future therapeutic delivery.
5. Discussion
The ability to turn back the biological clock at a cellular and tissue level has long been a pursuit confined to science fiction, yet the advent of transient reprogramming has brought this vision tantalizingly close to reality. Our review highlights the profound and multifaceted evidence supporting the multi-omic rejuvenation of naturally aged tissues through a single cycle of transient expression of Yamanaka factors. This paradigm represents a significant departure from full reprogramming, carefully balancing the induction of a youthful state with the maintenance of cellular identity, thereby avoiding the tumorigenic risks that have historically hampered direct therapeutic applications of pluripotency, a crucial lesson from cancer stem cell research.

The core strength of this approach lies in its multi-omic impact. At the epigenetic level, the consistent reversal of the epigenetic clock in various aged cell types and tissues is a powerful indicator of biological age reversal. This global epigenetic reset likely underpins the subsequent changes observed at the transcriptomic and proteomic levels, where age-associated gene expression patterns are reversed, and cellular machinery for protein maintenance and metabolism is restored to a more youthful state. Critically, these molecular shifts translate directly into tangible functional improvements in aged tissues. The reversal of fibrosis in kidneys, enhanced wound healing, and improved muscle regeneration collectively demonstrate that transient reprogramming is not merely a superficial molecular change but a deep, functional rejuvenation. This process effectively combats key hallmarks of aging, notably reducing the burden of cellular senescence and thereby mitigating chronic inflammation.
A central concern with any reprogramming strategy is safety, specifically the risk of dedifferentiation and uncontrolled proliferation leading to teratoma formation, a critical consideration directly informed by cancer stem cell research. The transient nature of the OSKM expression is the key to mitigating this risk. By limiting the duration of factor exposure, cells are guided toward a rejuvenated state without losing their tissue-specific identity. This delicate balance, the "sweet spot" of reprogramming, is a testament to meticulous experimental design and is crucial for eventual clinical translation. Continued research is vital to precisely define this optimal window and to identify the minimal set of factors or non-genetic interventions required to achieve rejuvenation with maximal safety.
The therapeutic potential of multi-omic rejuvenation for age-related diseases is immense. By addressing the fundamental processes of aging, this approach offers the promise of treating diseases at their root cause, rather than merely managing symptoms. Imagine a future where age-related neurodegeneration, cardiovascular dysfunction, or organ fibrosis could be ameliorated or even reversed by briefly turning back the biological clock of affected tissues. This could revolutionize the treatment landscape for a vast array of conditions that currently have limited effective therapies. The insights gained from how transient reprogramming remodels the epigenome also hold significant implications for epigenetic cancer therapies. Both fields manipulate epigenetic marks to alter cell fate or function; thus, advancements in one area could inform therapeutic strategies in the other, potentially leading to novel combination approaches or repurposing of epigenetic drugs.
Translational challenges, however, are significant. Developing safe and efficient in vivo delivery mechanisms for the reprogramming factors is paramount. While viral vectors have proven effective in preclinical models, concerns regarding immunogenicity and insertional mutagenesis necessitate the exploration of alternative, non-viral approaches. This is where the advancements in nanotechnology in oncology provide valuable precedents. The success of mRNA encapsulated in lipid nanoparticles (LNPs) for vaccine delivery demonstrates the feasibility of precise and transient in vivo genetic payload delivery. Adapting these nanotechnology platforms for targeted delivery of reprogramming factors to specific aged tissues or cell types would be a critical step towards clinical application. Future research also needs to focus on long-term safety profiles, the optimal frequency of transient reprogramming cycles, and identifying non-genetic or small-molecule mimetics of the reprogramming factors to enhance feasibility and reduce complexity. Furthermore, the ethical considerations surrounding human rejuvenation must be carefully addressed as the science progresses.
6. Conclusion
The paradigm of multi-omic rejuvenation through a single cycle of transient reprogramming represents a groundbreaking advancement at the intersection of aging research and regenerative medicine. By meticulously fine-tuning the expression of Yamanaka factors, scientists have demonstrated the unprecedented ability to reverse biological age across epigenetic, transcriptomic, and proteomic landscapes in naturally aged tissues, leading to tangible functional improvements. This approach skillfully navigates the crucial safety concerns inherited from cancer stem cell research by avoiding complete pluripotency and tumorigenesis. While significant translational challenges remain, particularly concerning safe and efficient in vivo delivery, where insights from nanotechnology in oncology can prove invaluable, this research fundamentally alters our understanding of aging plasticity. It opens revolutionary avenues for the prevention and treatment of age-related diseases, moving beyond symptomatic management to fundamentally reset cellular and tissue health. The continued exploration of this fascinating mechanism, including its overlap with principles relevant to epigenetic cancer therapies, promises to unlock the full potential of biological age reversal, ushering in an era of enhanced health span and unprecedented therapeutic opportunities.
Read more such content on @ Hidoc Dr | Medical Learning App for Doctors
Recommended News For You
Recommended Articles For You
Featured News
Featured Articles
Featured Events
Featured KOL Videos
1.
Coffee, Tea Tied to Lower Risk of Head and Neck Cancer
2.
PFS in Multiple Myeloma Is Improved by Daratumumab Added to Standard Therapy.
3.
A new drug delivery system may help patients with a rare eye cancer
4.
Traveling to Die: The Latest Form of Medical Tourism
5.
Clinical trial results show low-intensity therapy can achieve positive outcomes for certain pediatric leukemia subtypes
1.
Unexplained Weight Loss: Revealing Occult Cancers and Paraneoplastic Syndromes
2.
Exploring the Causes and Treatment of Granulocytopenia: A Comprehensive Guide
3.
HCC Codes in Oncology: Care Optimization in Plexiform Neurofibroma Management
4.
Multi-Omic Rejuvenation of Aged Tissues With Transient Reprogramming and Its Therapeutic Potential
5.
Respiratory Ramifications of Systemic Disease: A Comprehensive Review
1.
International Lung Cancer Congress®
2.
Future NRG Oncology Meeting
3.
Genito-Urinary Oncology Summit 2026
4.
ISMB 2026 (Intelligent Systems for Molecular Biology)
5.
Annual International Congress on the Future of Breast Cancer East
1.
Molecular Contrast: EGFR Axon 19 vs. Exon 21 Mutations - Part VI
2.
An Eagles View - Evidence-based Discussion on Iron Deficiency Anemia- Panel Discussion IV
3.
A Continuation to The Evolving Landscape of First-Line Treatment for Urothelial Carcinoma
4.
Evolving Space of First-Line Treatment for Urothelial Carcinoma- Case Discussion
5.
Treatment Sequencing Strategies in ALK + NSCLC Patients with CNS Diseases - Part II
© Copyright 2025 Hidoc Dr. Inc.
Terms & Conditions - LLP | Inc. | Privacy Policy - LLP | Inc. | Account Deactivation

